CBD Application Methods
How to choose the right CBD Product.
CBD products come in many different types, shapes, sizes, and concentrations.
If you are looking to try CBD for the first time it might be worthwhile knowing what to look for before selecting your CBD product. The efficacy of CBD products is greatly dependent on the type of application method and CBD concentration. The World Health Organization has stated that CBD is generally well-tolerated and with a good safety profile. Still, it is crucial to look into the actual concentration of CBD and make sure that the efficacy and safety profile of your chosen CBD product is well documented and reliable. If you are pregnant or under medication it is recommended to consult your doctor.
When looking into the different CBD products first you must decide which application method is right for you.
CBD Application Methods
1. Topical/ Transdermal
On the skin
2. Oral
Through the mouth
3. Nasal
Through the nose
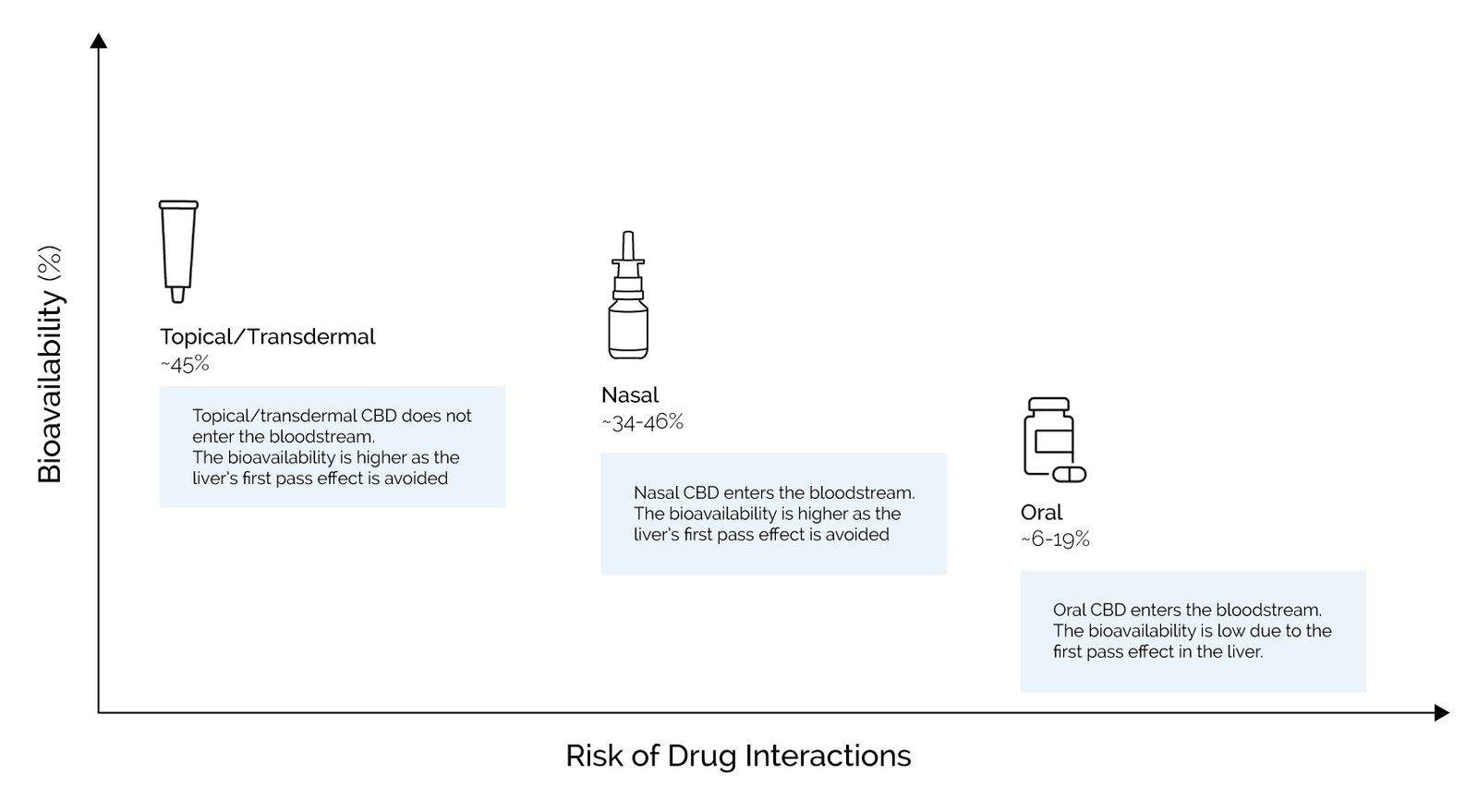
Application Methods & Efficiency
The 3 application methods have varying bioavailability & efficiency.
Bioavailability of CBD, the percentage of the used CBD that works in the body, varies greatly between application methods; Topical/transdermal (on the skin), Oral (through the mouth), and Nasal (through the nose). The difference in bioavailability between these methods has a direct impact on the effectiveness of the treatment.
Some studies show that CBD oral bioavailability ranges from 13-19% while other studies find that oral bioavailability is approximately 6% (dogs: up to 33%).1,2 Topical/transdermal and nasal products have a bioavailability of approximately 45% and 34-46%, respectively2,3 – making these applications the preferred ones.
Bioavailability
CBD bioavailability can differ depending on the method of application and CBD concentration in the product.
Bioavailability of an administered drug is always lower than 100%, as the body is not able to use all the administered drug. In the case of CBD products, this is caused by a process in our body called the first pass effect where CBD is broken down in the liver. Furthermore, gender, weight, health, and metabolism can all influence how well you respond to CBD and how long time it takes for it to work. Depending on the desired treatment some people may notice the desired results from CBD within a matter of days, while others may require weeks or even months of consistent use before beginning to notice a difference.
Oral CBD

Products
CBD oil, CBD Gummies, and CBD Tablets.
Path
Digestive process – meaning the CBD must pass through the liver before reaching the bloodstream.
Bioavailability
6 – 19% 1,2
First pass effect
CBD enters the liver, where it is broken down or changed to a large extent, drastically reducing the bioavailability, making it subtherapeutic.
Drug interactions
Oral CBD products pass the liver, causing a peak liver concentration which increases the risk for drug interactions.
Nasal CBD
Products
CBD Nose Sprays.
Path
The high vascularity in the nose allows CBD to go directly into the bloodstream.
Bioavailability
34-46% 2
First pass effect
Nasal CBD is quickly taken up into the blood, thereby avoiding the first-pass effect in the liver4 allowing more CBD to reach its target, and obtain the desired therapeutic effect.
Drug interactions
Within CBD being taken up quickly into the blood and not passing the liver first, the drug interactions are reduced to a minimum.
Topical CBD

Products
CBD Lotions, CBD Transdermal Patches and CBD Gels.
Path
Works locally (e.g., skin and joints) without entering the bloodstream because of the skin´s low permeability.
Bioavailability
First pass effect
Topical CBD does not pass the liver, thereby avoiding the first-pass effect, allowing more CBD to reach its target and maintain the desired therapeutic effect.
Drug interactions
Topical CBD products do not enter the bloodstream or liver, reducing the risk for drug interactions to a minimum.
REFERENCES
- Giacoppo, S., Galuppo, M., Pollastro, F., Grassi, G., Bramanti, P. & Mazzon, E. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. DARU, J. Pharm. Sci. 23, 1–17 (2015).
- Paudel, K. S., Hammell, D. C., Agu, R. U., Valiveti, S. & Stinchcomb, A. L. Cannabidiol bioavailability after nasal and transdermal application: Effect of permeation enhancers. Drug Dev. Ind. Pharm. 36, 1088–1097 (2010).
- Kazmira. Why Bioavailability Matters for CBD. at <https://www.kazmira-llc.com/homepage/blog/why-bioavailability-matters-for-cbd/>
- Bruni, N., Pepa, C. Della, Oliaro-Bosso, S., Pessione, E., Gastaldi, D. & Dosio, F. Cannabinoid delivery systems for pain and inflammation treatment. Molecules 23, (2018).
